12 new medicines recommended for approvalEMA’s human medicines committee (CHMP) recommended 12 medicines for approval at its March 2024 meeting.The CHMP recommended granting a…, Positive recommendations on new medicines , AwiqliInternational non-proprietary name (INN)insulin icodecMarketing-authorisation applicantNovo Nordisk A/STherapeutic indicationTreatment of diabetes mellitus in adults.More…, Emblaveo INNaztreonam / avibactamMarketing-authorisation applicantPfizer Europe Ma EEIGTherapeutic indicationTreatment of complicated Intra-Abdominal Infection (cIAI), complicated…, FabhaltaINNiptacopanMarketing-authorisation applicantNovartis Europharm LimitedTherapeutic indicationTreatment of paroxysmal nocturnal haemoglobinuria.More informationFabhalta:…, LytenavaINNbevacizumab Marketing-authorisation applicantOutlook Therapeutics LimitedTherapeutic indicationTreatment of neovascular (wet) age-related macular degeneration (…, Positive recommendation on new biosimilar medicines , JubbontiINNdenosumabMarketing-authorisation applicantSandoz GmbHTherapeutic indicationTreatment of osteoporosis.More informationJubbonti: Pending EC decision, Omlyclo INNomalizumabMarketing-authorisation applicantCelltrion Healthcare Hungary Kft.Therapeutic indicationTreatment of asthma.More informationOmlyclo: Pending EC decision, Wyost INNdenosumabMarketing-authorisation applicantSandoz GmbHTherapeutic indicationPrevention of skeletal related events with advanced malignancies.More informationWyost: Pending…, Positive recommendations on new hybrid medicines , AgilusINNdantrolene sodium, hemiheptahydrateMarketing-authorisation applicantNorgine B.V.Therapeutic indicationIn combination with adequate support measures, Agilus is indicated…, NeoatriconINNdopamine hydrochlorideMarketing-authorisation applicantBrePco Biopharma LimitedTherapeutic indicationTreatment of hypotension in neonates, infants and children.More…, Positive recommendations on new generic medicines , Dimethyl fumarate Accord INNdimethyl fumarateMarketing-authorisation applicantAccord HealthcareTherapeutic indicationFor the treatment of adult and paediatric patients aged 13…, Dimethyl fumarate MylanINNdimethyl fumarateMarketing-authorisation applicantMylan Ireland LimitedTherapeutic indicationFor the treatment of adult and paediatric patients aged 13…, Dimethyl fumarate Neuraxpharm INNdimethyl fumarateMarketing-authorisation applicantNeuraxpharm Pharmaceuticals S.L.Therapeutic indicationFor the treatment of adult and paediatric…, Positive recommendations on extensions of indications , BimzelxINNbimekizumabMarketing-authorisation holderUCB Pharma S.AMore informationBimzelx: Pending EC decision, NilemdoINNbempedoic acid Marketing-authorisation holderDaiichi Sankyo Europe GmbHMore informationNilemdo: Pending EC decision, NustendiINNbempedoic acid / ezetimibe Marketing-authorisation holderDaiichi Sankyo Europe GmbHMore informationNustendi: Pending EC decision, Onivyde pegylated liposomalINNirinotecan hydrochloride trihydrateMarketing-authorisation holderLes Laboratoires ServierMore informationOnivyde pegylated liposomal: Pending EC…, RetsevmoINNselpercatinibMarketing-authorisation holderEli Lilly Nederland B.V.More informationRetsevmo: Pending EC decision, XtandiINNenzalutamideMarketing-authorisation holderAstellas Pharma Europe B.V.More informationXtandi: Pending EC decision, Withdrawal of applications to change the marketing authorisation , AdcetrisINNbrentuximab vedotinMarketing-authorisation holderTakeda Pharma A/SMore informationAdcetris: Withdrawn application, OngentysINNopicaponeMarketing-authorisation holderBial Portela & Companhia S.A.More informationOngentys: Withdrawn application, OntilyvINNopicaponeMarketing-authorisation holderBial Portela & Companhia S.A.More informationOntilyv: Withdrawn application, OrenciaINNabataceptMarketing-authorisation holderBristol-Myers Squibb PharmaMore informationOrencia: Withdrawn application, Outcome of arbitration procedures , MicrazymINNporcine pancreas enzymesMarketing-authorisation applicanAvva Pharmaceuticals Ltd.More informationMicrazym, Re-examinations of referral procedure , Synapse Labs Pvt. Ltd.More informationSynapse - referral
Source: EMA
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 March 2024
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 18-21 March 2024
投稿日:






















































 2008年11月、metzdowd.comにナカモトサトシにより投稿された論文
2008年11月、metzdowd.comにナカモトサトシにより投稿された論文 ブロックチェーン
ブロックチェーン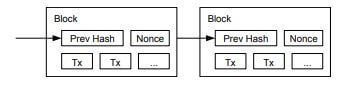
 ビットコインは送信アドレス(Tx)に対するデジタル署名によって保護されており、一定時間(10分)ごとに、すべての取引記録を分散台帳に追加します。
ビットコインは送信アドレス(Tx)に対するデジタル署名によって保護されており、一定時間(10分)ごとに、すべての取引記録を分散台帳に追加します。