Guideline on safety and residue data requirements for applications for non-immunological veterinary medicinal products intended for limited markets but not eligible for authorisation under Article 23 of Regulation (EU) 2019/6
Source: EMA
Guideline on safety and residue data requirements for applications for non-immunological veterinary medicinal products intended for limited markets but not eligible for authorisation under Article 23 of Regulation (EU) 2019/6
Guideline on safety and residue data requirements for applications for non-immunological veterinary medicinal products intended for limited markets but not eligible for authorisation under Article 23 of Regulation (EU) 2019/6
投稿日:






















































 2008年11月、metzdowd.comにナカモトサトシにより投稿された論文
2008年11月、metzdowd.comにナカモトサトシにより投稿された論文 ブロックチェーン
ブロックチェーン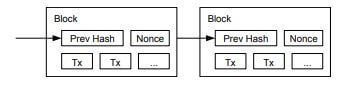
 ビットコインは送信アドレス(Tx)に対するデジタル署名によって保護されており、一定時間(10分)ごとに、すべての取引記録を分散台帳に追加します。
ビットコインは送信アドレス(Tx)に対するデジタル署名によって保護されており、一定時間(10分)ごとに、すべての取引記録を分散台帳に追加します。